Introduction
Ammonia (NH3) is a chemical compound consisting of nitrogen and hydrogen, with a pungent odor that’s easily recognizable. Widely known for its extensive industrial applications, ammonia plays a crucial role in several sectors, including agriculture, refrigeration, and manufacturing.
What is Ammonia?
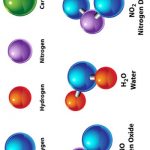
Ammonia is a simple molecule composed of one nitrogen atom (N) and three hydrogen atoms (H). The chemical formula for ammonia is NH3. It’s a gas at room temperature and is highly soluble in water. Ammonia is used primarily as a building block for creating various nitrogen-containing compounds.
Ammonia’s Structure and Properties
Ammonia has a trigonal pyramidal molecular structure. The NH3 molecule consists of nitrogen at the centre and three hydrogen atoms at the corners. Ammonia is a polar molecule, meaning it has a partial positive and negative charge, which influences how it reacts with other substances. Its boiling point is approximately −33.35 °C, and it solidifies at −77.7 °C.
What is Ammonia Made Of?
Ammonia combines nitrogen (N) and hydrogen (H) atoms. In nature, ammonia is produced during the decomposition of organic matter. At the same time, industrially, it is synthesized via the Haber-Bosch process, where nitrogen gas reacts with hydrogen under high pressure and temperatures.
Ammonia in Chemistry
Chemically, ammonia is known for its basic properties due to a lone pair of electrons on the nitrogen atom. In aqueous solutions, it forms ammonium hydroxide (NH4OH). Ammonia is often referred to by its chemical name or its common industrial shorthand NH3.
ammonia solutions
At DSW, we offer a wide range of ammonia solutions in various purity levels and concentrations to meet your industrial needs. Contact us today to find the perfect ammonia product for your application!
Uses of Ammonia
Fertilizers
The most prominent use of ammonia is in agriculture. Ammonium nitrate (NH4NO3) and urea are derived from ammonia and are widely used as fertilizers. The compound delivers essential nitrogen, promoting plant growth.
Industrial Refrigeration
Ammonia is a key component in refrigeration systems due to its excellent heat absorption properties. Its use as a coolant in industrial refrigeration systems is widespread, especially in large-scale operations like food processing.
Chemical Reactions and Compounds
Ammonia is involved in numerous chemical reactions, forming compounds such as hydrazine (N2H4), which is used in rocket fuel, and hydroxylamine (NH2OH), a chemical intermediate used in various syntheses. Ammonia derivatives are essential in producing chemicals for multiple industries.
Biological Definition of Ammonia
In biological terms, ammonia is a byproduct of protein metabolism in organisms. It is toxic in high concentrations but is typically converted to urea or uric acid, depending on the species, for safe excretion.
Ammonia as a Compound
Ammonia is a compound, specifically a covalent compound due to the sharing of electrons between nitrogen and hydrogen atoms. It’s a stable molecule that can participate in both acid-base reactions and oxidation-reduction reactions.
What is NH3 in Chemistry?
In chemistry, NH3 represents ammonia, a critical molecule for nitrogen fixation and as a precursor for many synthetic chemicals. When dissolved in water, NH3 forms ammonium (NH4+) ions, a key player in biological systems and agricultural processes.
Reactions of Ammonia
Ammonia participates in various reactions, such as:
- NH3 Reaction: Combustion of ammonia produces nitrogen gas and water.
- Acid-Base Reactions: Ammonia acts as a base and reacts with acids to form ammonium salts.
- Redox Reactions: Ammonia can act as a reducing agent in the presence of oxygen.
Ammonia’s Chemical Formula and Derivatives
The chemical formula for ammonia is NH3, and it has various derivatives, including:
- Hydrazine (N2H4): A fuel component.
- Ammonium salts: Used in fertilizers and other applications.
Ammonia: Is it an Acid or Base?
Ammonia is classified as a weak base. When dissolved in water, it forms ammonium hydroxide, making the solution essential, though less so than solid bases like sodium hydroxide (NaOH).
Conclusion
Ammonia (NH3) is a vital chemical compound with diverse applications ranging from agriculture to industrial processes. Whether used as a fertilizer, in refrigeration, or in chemical manufacturing, its utility and importance cannot be overstated. Understanding ammonia’s structure, chemical behavior, and derivatives provides insights into why it remains a cornerstone of modern industry.










No comment