What Is Nitrous Oxide?
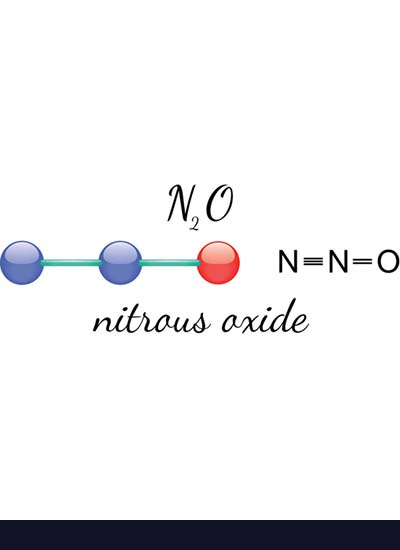
Nitrous oxide, also called laughing gas, has a sweet smell and taste. It is used for dentistry and surgery, producing inhaled analgesics and anesthetics. N2o gas is used in rocketry and to increase the performance of automobile engines. Inhaling nitrous oxide produces euphoria, hence the term “laughing gases.”
Manufacturing of Nitrous Oxide
-
- The production process of N2O can be relatively straightforward. Ammonium Nitrate (NH4NO3) is available as liquid ammonium (LAN) or solid pellets (SAN). This material is used as a fertilizer and also as an explosive.
- The NH4NO3 is heated to 250deg C, decomposing (NH4NO3-N2O+2H2O) into N2O and water (steam). The mixture of gas and water is cooled down to room temperature. Steam is then condensed, and the majority of the water is removed. The crude mixture of N2O is then scrubbed to remove contaminants. The gas mixture is compressed, dried, cooled, and liquefied. The resultant product is almost pure (99.5%-99.9%), stored in a liquefied compressed gas in tanks that are insulated and chilled at 300 psi.
B. Application of Nitrous oxide
1. Major manufacturers produce About 90% of N2O in the health sector. This amount (80%-85%) is mainly used in hospitals for general anesthesia. In ambulatory dental clinics, up to 10% N2O is used.
2. Food industry uses between 5% and 8% of N2O produced. N2O is used as a fuel in dairy products, such as whipped cream. Nitrous oxide creates a cream four times as thick as the liquid. Nitrous oxide, being lipophilic, inhibits the degradation of the fat cream. If pressurized oxygen were used, it would cause the cream’s rancidity. Nitrous oxide is also used to replace oxygen in food packages such as potato chip bags to prevent crushing and prolong the freshness.
3. The chemical industry is one of the remaining users of Nitrous Oxide. In this case, nitrous dioxide produces sodium azide, an explosive agent in automobile airbags.
4. In the racing industry, N2O can improve engine performance (cars, motorcycles, and boats). Now, N2O is available for personal vehicles.
5. In the computer chip manufacturing industry, N2O oxidizes chemicals. For this use, the product must be 100% pure.
6. In hybrid rocket engines, Nitrous Oxide is used in conjunction with fuel as an oxidizer. Also, it has been used for amateur and high-power rocketry.
Managing Chemical Safety in the Workplace – N2O GAS
Nitrous oxide is a colorless liquid gas. Inhaling nitrous dioxide can cause nausea, dizziness, and even death. Infertility can be caused by long-term exposure. Contact with liquid nitrogen oxide can lead to severe frostbite. Exposure to nitrous dioxide can cause harm to workers. The direction depends on the work’s type, duration, and dose.
Nitrous oxide can be found in many industries. It can be produced by anesthetic devices, surgical patients, and storage cylinders. Workers at risk for nitrous dioxide exposure include:
Personnel who works in the operating rooms of hospitals, surgery centers, or medical offices
Dental workers who use nitrous dioxide gas as an anesthetic
Outgassing is a risk to recovery room staff, which may be exposed to patients who have had surgery and are emitting fumes.
Workers in laboratories or facilities that store and move compressed gas cylinders
Cleaning and maintaining surgical rooms before or after surgery is done by service workers.
Regulation and Control of Nitrous Oxide
1. The Food and Drug Administration (FDA) regulates the N2O industry. The FDA has established good manufacturing practices (GMPs) and quality system requirements (QSRs) for companies producing and packaging gases. Compliance with these regulations results in a nearly 100% pure product exceeding U.S. Pharmacopeia specifications.
2. In addition, the U.S. Department of Transportation (DOT) oversees the packaging and transport of gas. N2O is considered a hazardous material because of the pressurization. Therefore, it falls under the restrictions of the DOT Hazardous Materials Regulations. Cylinders, vehicles, and drivers must meet prescribed regulations







No comment